This Week in Neuroscience and Psychedelics
Playing a clarinet while receiving brain surgery? Yep, it really happened. Plus the reason Alto Neuroscience's share price is on the rise.
This Week...
Alto Neuroscience got a nice bump in share price this week after the company announced it was accelerating the development of its treatment resistant depression treatment, ALTO-207, following a successful meeting with the FDA.
Alto Neuroscience is a clinical-stage biopharmaceutical company focused on developing novel precision medicines for neuropsychiatric disorders. And ALTO-207 is a fixed-dose combination of pramipexole, a dopamine D3-preferring D3/D2 agonist approved for the treatment of Parkinson’s disease with demonstrated antidepressant effect, and ondansetron, an antiemetic selective 5-HT3 receptor antagonist.
The same day Alto announced it was accelerating its development of ALTO-207, company reps also announced a $50 million private placement which will be used to expand development of ALTO-207. Alto now expects to initiate a Phase 3 study by early 2027. Here’s more: https://investors.altoneuroscience.com/news/news-details/2025/Alto-Neuroscience-Announces-Plans-to-Accelerate-Development-of-ALTO-207-in-Treatment-Resistant-Depression-Following-Successful-Outcome-from-Recent-FDA-Meeting/default.aspx
Clearmind Medicine announced that it enrolled the final patient for the first cohort of its Phase I/IIa clinical trial evaluating CMND-100, which is the company’s proprietary MEAI-based oral drug candidate for the treatment of Alcohol Use Disorder (AUD).
The multinational, multicenter Phase I/IIa trial is designed as a single- and multiple-dose study to assess the safety, tolerability, and pharmacokinetic profile of CMND-100. It will also explore preliminary efficacy signals, such as reductions in alcohol cravings and consumption, among participants who are either non-treatment-seeking individuals reporting heavy binge drinking or treatment-seeking individuals diagnosed with AUD. Check it out: https://finance.yahoo.com/news/clearmind-medicine-enrolls-last-patient-112500819.html
The FDA selected nine new companies to participate in its new CNPV program. This pilot program, initiated by the FDA, is designed to accelerate the regulatory review of certain drugs or biologics that align with specific U.S. national health-and-security priorities. One of those companies is pharmaceutical manufacturer Phlow Corporation, which proposed ketamine for the CNPV program.
Here’s what CEO Dr. Eric Edwards had to say about the FDA’s decision…
We are honored to have been selected to participate in the FDA Commissioner’s National Priority Voucher pilot program. This critical initiative will accelerate our efforts to support domestic production of the active pharmaceutical ingredient for ketamine, further strengthening resilience in the U.S. supply chain.
You can read more about the deal here: https://substack.com/home/post/p-176840785
Did You Know?
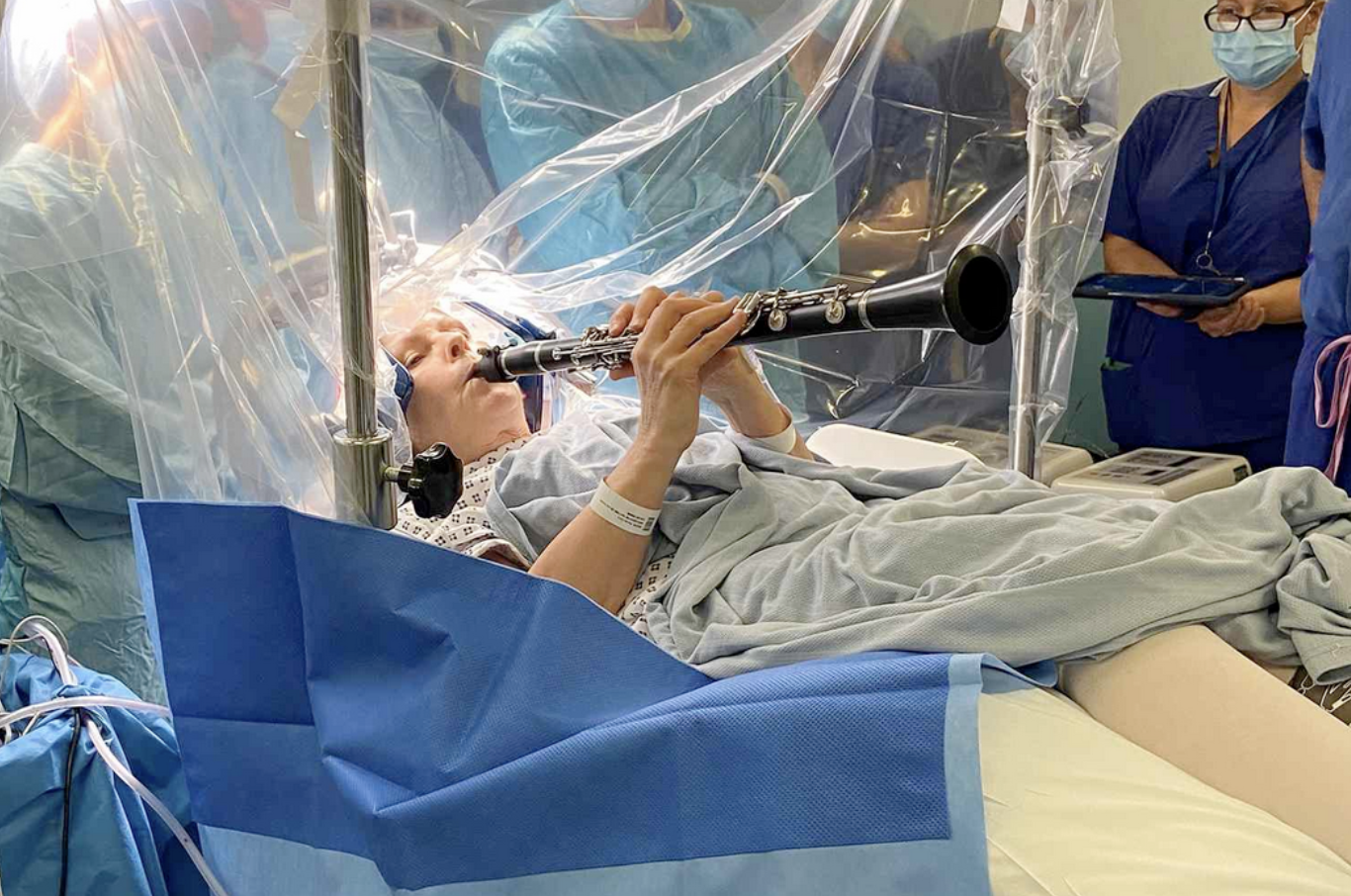
Did you know that a woman with Parkinson’s recently played her clarinet while undergoing brain surgery?
During a four-hour operation at London's King's College Hospital, Denise Bacon played her clarinet while Keyoumars Ashkan, a professor of neurosurgery, performed the procedure to help reduce her symptoms, allowing for immediate improvement in her finger movements.
The professor said she played the clarinet continuously, which helped to "fine tune the position of the electrodes deep inside her brain, until she was able to play her beloved musical instrument." Absolutely amazing! Check it out: https://www.bbc.com/news/articles/cq83k7l7jyyo